Novel Foam-Based Design Demonstrates Successful LAAO Seal with No Device-Related Thrombus
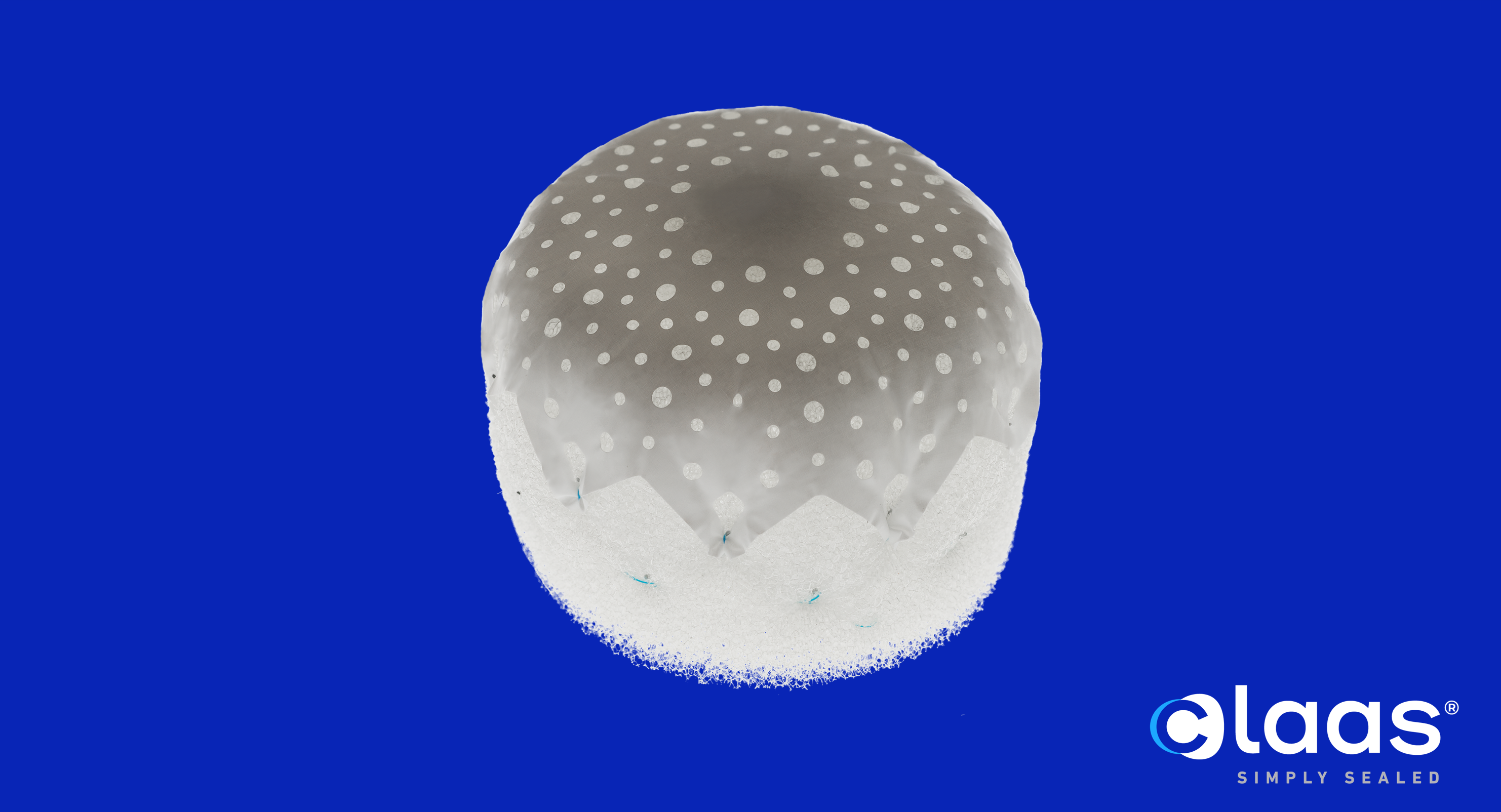
Conformal Medical, Inc., a medical device company developing next-generation left atrial appendage occlusion (LAAO) technology, today announced the publication of the peer-reviewed article, “Foam-based Left Atrial Appendage Closure (CLAAS) Device: Evaluation in a Chronic Canine Model,” in the Journal of the Society for Cardiovascular Angiography and Interventions (JSCAI). This publication summarizes a pre-clinical study demonstrating key features of the CLAAS Device including the ability to position, deploy and seal the LAA, with appropriate healing at 150 days in a healthy canine model.
“This study validates the design features of Conformal’s CLAAS foam-based implant that enable it to fill in gaps and definitively seal, regardless of the left atrial appendage (LAA) shape,” stated William Gray, MD, Chief of Cardiovascular Disease at Main Line Health and co-Director of Lankenau Heart Institute in Wynnewood, PA.
The CLAAS System is designed to seal the LAA in patients with non-valvular atrial fibrillation (Afib) to reduce the risk of stroke without the need for anticoagulants. Featuring a proprietary foam-based architecture, the CLAAS implant is designed to conform to a broad range of LAA anatomies with only two sizes and aims to simplify the procedure by allowing off-axis positioning while providing a more uniform seal. The implant also aims to eliminate the need for procedural transesophageal echocardiogram so that physicians may perform the procedure without general anesthesia, a significant advancement with the potential to shift clinical practice to a single operator procedure performed under conscious sedation without general anesthesia.
“We are pleased to have these data published, demonstrating the ability of the CLAAS implant to successfully close the LAA in a healthy canine model with normal local healing without signs of device related thrombus, thrombus embolization, infection, or toxicity,” commented Aaron Kaplan, MD, Co-Founder and Chief Medical Officer for Conformal Medical. “We look forward to further validating its performance in the clinic as we accelerate enrollment in the CONFORM pivotal trial.”
Conformal Medical is actively enrolling patients in the CONFORM pivotal trial, evaluating the safety and efficacy of the CLAAS® System compared to other commercially available LAAO devices. The prospective, multicenter, randomized controlled study will enroll approximately 1,600 patients in the U.S. and Japan and will support U.S. Food and Drug Administration (FDA) pre-market approval.
About Conformal Medical
Founded in 2016, Conformal Medical, Inc. is a medical device company developing devices to prevent stroke in patients with non-valvular Afib. The company’s proprietary technology is intended to make LAAO a same day, single operator procedure. For more information, visit https://conformalmedical.com/.
About Left Atrial Appendage Closure
More than six million people in the United States suffer from Afib, placing them at an increased risk of stroke.1 Current standard of care for stroke prevention is chronic oral anticoagulants, which are not well accepted by patients due to concern about associated risk of bleeding. LAAO is emerging as an important alternative to blood thinners for preventing strokes in patients with non-valvular Afib. In 2021, the global LAAO market was estimated at $900M and is expected to exceed $2B in 2024.2,3
MEDIA CONTACT:
Katie Arnold
SPRIG Consulting LLC
408-805-0520
katie@sprigconsulting.com
1. Kornej J, Börschel CS, Benjamin EJ, Schnabel RB. Epidemiology of Atrial Fibrillation in the 21st Century: Novel Methods and New Insights. Circ Res. 2020 Jun 19;127(1):4-20.
2. Boston Scientific Investor Presentation, September 2021.
3. Key Takeaways from the SVB Leerink Global Healthcare Conference, February 2022.
